Is Hormone Therapy Linked to Breast Cancer in Seniors Peer Reviews
What are hormones and hormone receptors?
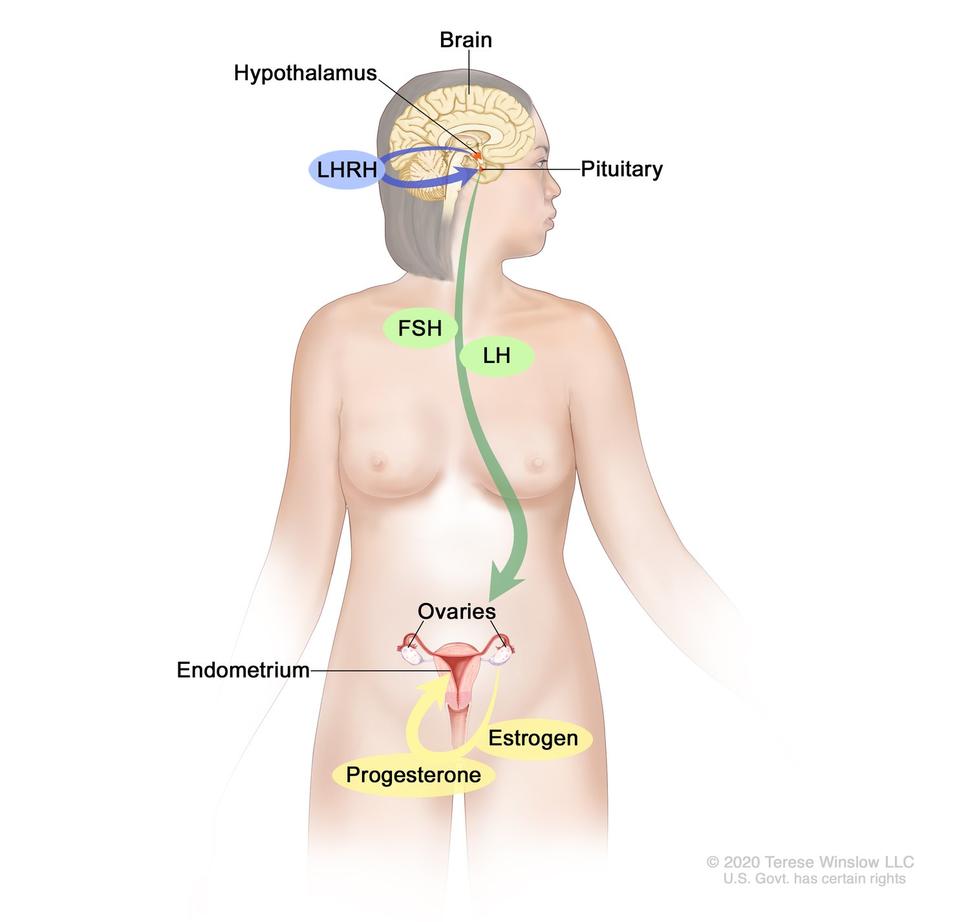
Hormones are substances that role as chemical messengers in the torso. They touch on the actions of cells and tissues at various locations in the body, often reaching their targets through the bloodstream.
The hormones estrogen and progesterone are produced past the ovaries in premenopausal women and past some other tissues, including fat and pare, in both premenopausal and postmenopausal women and in men. Estrogen promotes the development and maintenance of female person sex characteristics and the growth of long bones. Progesterone plays a role in the menstrual cycle and pregnancy.
Estrogen and progesterone also promote the growth of some breast cancers, which are called hormone-sensitive (or hormone-dependent) breast cancers. Hormone-sensitive breast cancer cells contain proteins chosen hormone receptors (estrogen receptors, or ERs, and progesterone receptors, or PRs) that become activated when hormones bind to them. The activated receptors cause changes in the expression of specific genes, which can stimulate cell growth.
To determine whether chest cancer cells incorporate hormone receptors, doctors test samples of tumor tissue that have been removed by surgery. If the tumor cells contain estrogen receptors, the cancer is called estrogen receptor positive (ER positive), estrogen sensitive, or estrogen responsive. Similarly, if the tumor cells contain progesterone receptors, the cancer is called progesterone receptor positive (PR or PgR positive). Breast tumors that contain estrogen and/or progesterone receptors are sometimes called hormone receptor positive (HR positive). Well-nigh ER-positive breast cancers are also PR positive.
Chest cancers that lack ERs are called ER negative, and if they lack both ER and PR they may exist chosen HR negative.
Approximately 67%–80% of chest cancers in women are ER positive (1, 2). Approximately xc% of breast cancers in men are ER positive and approximately 80% are PR positive (3).
What is hormone therapy?
Hormone therapy (also called hormonal therapy, hormone treatment, or endocrine therapy) slows or stops the growth of hormone-sensitive tumors by blocking the body's ability to produce hormones or by interfering with effects of hormones on chest cancer cells. Tumors that are hormone insensitive practise not have hormone receptors and do not reply to hormone therapy.
Hormone therapy for breast cancer should not be confused with menopausal hormone therapy (MHT)—treatment with estrogen lonely or in combination with progesterone to assist relieve symptoms of menopause. These two types of therapy produce opposite effects: hormone therapy for breast cancer blocks the growth of 60 minutes-positive breast cancer, whereas MHT tin can stimulate the growth of Hour-positive breast cancer. For this reason, when a adult female taking MHT is diagnosed with HR-positive breast cancer she is commonly asked to cease that therapy.
What types of hormone therapy are used for breast cancer?
Several strategies are used to treat hormone-sensitive breast cancer:
Blocking ovarian function: Because the ovaries are the main source of estrogen in premenopausal women, estrogen levels in these women can be reduced by eliminating or suppressing ovarian role. Blocking ovarian part is called ovarian ablation.
Ovarian ablation can be done surgically in an operation to remove the ovaries (called oophorectomy) or by treatment with radiation. This type of ovarian ablation is usually permanent.
Alternatively, ovarian part can be suppressed temporarily by handling with drugs called gonadotropin-releasing hormone (GnRH) agonists, which are also known every bit luteinizing hormone-releasing hormone (LHRH) agonists. Past mimicking GnRH, these medicines interfere with signals that stimulate the ovaries to produce estrogen.
Examples of ovarian suppression drugs that take been approved past the U.s. Food and Drug Administration (FDA) are goserelin (Zoladex) and leuprolide (Lupron).
Blocking estrogen production: Drugs called aromatase inhibitors are used to block the activity of an enzyme chosen aromatase, which the body uses to make estrogen in the ovaries and in other tissues. Aromatase inhibitors are used primarily in postmenopausal women because the ovaries in premenopausal women produce too much aromatase for the inhibitors to block effectively. Nevertheless, these drugs can be used in premenopausal women if they are given together with a drug that suppresses ovarian function.
Examples of aromatase inhibitors approved by the FDA are anastrozole (Arimidex) and letrozole (Femara), both of which temporarily inactivate aromatase, and exemestane (Aromasin), which permanently inactivates aromatase.
Blocking estrogen's effects: Several types of drugs interfere with estrogen's ability to stimulate the growth of breast cancer cells:
- Selective estrogen receptor modulators (SERMs) bind to estrogen receptors, preventing estrogen from binding. Examples of SERMs canonical by the FDA for treatment of breast cancer are tamoxifen (Nolvadex) and toremifene (Fareston).
Because they bind to estrogen receptors, SERMs can potentially non only block estrogen activeness (by preventing estrogen from binding to its receptor) but also mimic the effects of estrogen, depending on where they are expressed in the trunk. For case, tamoxifen blocks the effects of estrogen in breast tissue simply acts like estrogen in the uterus and bone.
- Other antiestrogen drugs, such as fulvestrant (Faslodex), work in a somewhat dissimilar way to cake estrogen's effects. Similar SERMs, fulvestrant binds to the estrogen receptor and functions as an estrogen blocker. However, unlike SERMs, fulvestrant does not mimic estrogen. For this reason, information technology is called a pure antiestrogen. In addition, when fulvestrant binds to the estrogen receptor, the receptor is targeted for destruction.
How is hormone therapy used to care for breast cancer?
There are iii main ways that hormone therapy is used to treat hormone-sensitive breast cancer:
Adjuvant therapy for early-stage chest cancer: Tamoxifen is FDA approved for adjuvant hormone handling of premenopausal and postmenopausal women (and men) with ER-positive early-stage chest cancer, and the aromatase inhibitors anastrozole, letrozole, and exemestane are approved for this utilise in postmenopausal women.
Research has shown that women who receive at least 5 years of adjuvant therapy with tamoxifen after having surgery for early-stage ER-positive breast cancer have reduced risks of breast cancer recurrence, including a new breast cancer in the other breast, and reduced run a risk of death at 15 years (four).
Until recently, most women who received adjuvant hormone therapy to reduce the chance of a chest cancer recurrence took tamoxifen every solar day for 5 years. Even so, with the introduction of newer hormone therapies (i.e., the aromatase inhibitors), some of which have been compared with tamoxifen in clinical trials, additional approaches to hormone therapy take become common (5–7).
For example, some women may take an aromatase inhibitor, instead of tamoxifen, every twenty-four hours for 5 years. Other women may receive additional handling with an aromatase inhibitor afterwards 5 years of tamoxifen. Finally, some women may switch to an aromatase inhibitor afterwards 2 or 3 years of tamoxifen, for a total of v or more years of hormone therapy. Inquiry has shown that for postmenopausal women who have been treated for early on-stage breast cancer, adjuvant therapy with an aromatase inhibitor reduces the run a risk of recurrence and improves overall survival compared with adjuvant tamoxifen (8).
Some premenopausal women with early-stage ER-positive breast cancer may take ovarian suppression plus an aromatase inhibitor, which was constitute to have higher rates of liberty from recurrence than ovarian suppression plus tamoxifen or tamoxifen alone (9).
Men with early on-stage ER-positive breast cancer who receive adjuvant therapy are usually treated first with tamoxifen. Those treated with an aromatase inhibitor usually too take a GnRH agonist.
Decisions well-nigh the type and duration of adjuvant hormone therapy are complicated and must be fabricated on an individual basis in consultation with an oncologist.
Treatment of advanced or metastatic breast cancer: Several types of hormone therapy are approved to treat metastatic or recurrent hormone-sensitive chest cancer. Hormone therapy is also a treatment option for ER-positive breast cancer that has come up back in the breast, chest wall, or nearby lymph nodes after handling (also called a locoregional recurrence).
Two SERMs, tamoxifen and toremifene, are approved to care for metastatic chest cancer. The antiestrogen fulvestrant is approved for postmenopausal women with metastatic ER-positive chest cancer that has spread later on treatment with other antiestrogens (x). Fulvestrant is besides approved for postmenopausal women with Hour-positive, HER2-negative locally advanced or metastatic breast cancer who accept non previously been treated with hormone therapy (11). In addition, it may exist used in premenopausal women who have had ovarian ablation.
The aromatase inhibitors anastrozole and letrozole are approved to exist given to postmenopausal women equally initial therapy for metastatic or locally advanced hormone-sensitive chest cancer (12, thirteen). Both of these drugs and the aromatase inhibitor exemestane are also approved to treat postmenopausal women with advanced breast cancer whose disease has worsened later handling with tamoxifen (xiv). Men with advanced breast cancer who are treated with an aromatase inhibitor too receive a GnRH agonist.
Some women with advanced breast cancer are treated with a combination of hormone therapy and one of several targeted therapies:
- Palbociclib (Ibrance), is approved for utilise in combination with letrozole as initial therapy for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer in postmenopausal women. Palbociclib inhibits two cyclin-dependent kinases (CDK4 and CDK6) that announced to promote the growth of HR-positive chest cancer cells (15).
Palbociclib is also canonical to exist used in combination with fulvestrant for the treatment of postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer whose cancer has gotten worse after treatment with another hormone therapy (16).
- Abemaciclib (Verzenio), another CDK4 and CDK6 inhibitor, is approved to be used in combination with fulvestrant for postmenopausal women with Hour-positive, HER2-negative advanced or metastatic chest cancer whose disease has progressed subsequently treatment with hormone therapy (17).
Abemaciclib is besides approved to be used lonely for women and men with 60 minutes-positive, HER2-negative advanced or metastatic breast cancer whose disease got worse after treatment with hormone therapy and previous chemotherapy given for metastatic disease (eighteen).
Abemaciclib is as well canonical to be used with an aromatase inhibitor every bit first-line hormone therapy in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer.
- Ribociclib (Kisqali), another CDK4/half-dozen inhibitor, is canonical to exist used in combination with an aromatase inhibitor in postmenopausal women with 60 minutes-positive, HER2-negative avant-garde or metastatic chest cancer that has not been treated with hormone therapy (19, twenty).
Ribociclib is too canonical to be used in combination with fulvestrant in postmenopausal women with HR-positive, HER2-negative avant-garde or metastatic breast cancer who have not been treated with hormone therapy or whose disease got worse during treatment with hormone therapy (21).
- Lapatinib (Tykerb) is approved to be used in combination with letrozole to treat 60 minutes-positive, HER2-positive metastatic chest cancer in postmenopausal women for whom hormone therapy is indicated. Information technology is a small-molecule inhibitor of the HER2 and EGFR tyrosine kinases.
- Alpelisib (Piqray) is approved to treat breast cancer that is Hour positive and HER2 negative and has a mutation in the PIK3CA gene. Information technology is used with fulvestrant to treat postmenopausal women, and men, whose chest cancer is advanced or metastatic and has gotten worse during or after treatment with hormone therapy (22).
- Some women with advanced chest cancer that is HER2 and Hour positive may receive hormone therapy plus trastuzumab with or without pertuzumab (23).
Neoadjuvant treatment of breast cancer: The use of hormone therapy to treat breast cancer to reduce tumor size before surgery (neoadjuvant therapy) has been studied in clinical trials (24). These trials take shown that neoadjuvant hormone therapy—in particular, with aromatase inhibitors—tin be effective in reducing the size of chest tumors in postmenopausal women, just it is not yet articulate how effective it is in premenopausal women.
Hormone therapy is sometimes used for the neoadjuvant treatment of HR-positive breast cancer in postmenopausal women who cannot tolerate chemotherapy or when surgery needs to be delayed.
Can hormone therapy exist used to prevent breast cancer?
Yes. Most breast cancers are ER positive, and clinical trials have tested whether hormone therapy can be used to forbid breast cancer in women who are at increased take a chance of developing the disease.
A large NCI-sponsored randomized clinical trial chosen the Breast Cancer Prevention Trial establish that tamoxifen, taken for 5 years, reduces the risk of developing invasive breast cancer by about 50% in postmenopausal women who were at increased adventure (25). Long-term follow-upwards of another randomized trial, the International Breast Cancer Intervention Study I, institute that v years of tamoxifen treatment reduces the incidence of breast cancer for at least 20 years (26). A subsequent large randomized trial, the Written report of Tamoxifen and Raloxifene, which was besides sponsored by NCI, plant that 5 years of raloxifene (a SERM) reduces breast cancer gamble in such women by near 38% (27).
As a consequence of these trials, both tamoxifen and raloxifene have been canonical past the FDA to reduce the risk of developing breast cancer in women at high risk of the illness. Tamoxifen is approved for this use regardless of menopausal condition. Raloxifene is approved for apply only in postmenopausal women.
2 aromatase inhibitors—exemestane and anastrozole—accept as well been constitute to reduce the hazard of breast cancer in postmenopausal women at increased risk of the illness. Later on iii years of follow-upward in a randomized trial, women who took exemestane were 65% less likely than those who took a placebo to develop chest cancer (28). Subsequently 7 years of follow-upwardly in another randomized trial, women who took anastrozole were l% less likely than those who took a placebo to develop breast cancer (29). Both exemestane and anastrozole are canonical by the FDA for treatment of women with ER-positive breast cancer. Although both are as well used for breast cancer prevention, neither is approved for that indication specifically.
What are the side furnishings of hormone therapy?
The side effects of hormone therapy depend largely on the specific drug or the blazon of handling (7). The benefits and harms of taking hormone therapy should exist carefully weighed for each person. A mutual switching strategy used for adjuvant therapy, in which patients have tamoxifen for two or 3 years, followed past an aromatase inhibitor for 2 or 3 years, may yield the best residue of benefits and harms of these ii types of hormone therapy (30).
Hot flashes, dark sweats, and vaginal dryness are mutual side furnishings of all hormone therapies. Hormone therapy also may disrupt the menstrual cycle in premenopausal women.
Less mutual but serious side effects of hormone therapy drugs are listed below.
Tamoxifen
- risk of claret clots, especially in the lungs and legs
- stroke
- cataracts
- endometrial cancer and uterine sarcoma
- bone loss in premenopausal women, but no increased risk of fracture
- mood swings, depression, and loss of libido
- in men: headaches, nausea, vomiting, pare rash, impotence, and loss of libido
Raloxifene
- adventure of claret clots, especially in the lungs and legs
- stroke in certain subgroups
Ovarian suppression
- bone loss
- mood swings, depression, and loss of libido
Aromatase inhibitors
- risk of centre attack, angina, centre failure, and hypercholesterolemia
- os loss
- joint pain
- mood swings and low
Fulvestrant
- gastrointestinal symptoms, including nausea, vomiting, and constipation
- weakness and fatigue
- hurting, including os hurting, back pain, musculoskeletal pain, joint pain, and in the extremities
- headache
- hot flashes
- animate problems, including painful breathing, shortness of breath, and cough
- loss of appetite
Can other drugs interfere with hormone therapy?
Sure drugs, including several ordinarily prescribed antidepressants (those in the category called selective serotonin reuptake inhibitors, or SSRIs), inhibit an enzyme called CYP2D6. This enzyme plays a critical part in the body'due south use of tamoxifen because CYP2D6 metabolizes, or breaks down, tamoxifen into molecules, or metabolites, that are much more active than tamoxifen itself.
The possibility that SSRIs might, by inhibiting CYP2D6, slow the metabolism of tamoxifen and reduce its effectiveness is a business organisation given that as many as ane-fourth of chest cancer patients experience clinical low and may be treated with SSRIs. In addition, SSRIs are sometimes used to care for hot flashes caused past hormone therapy.
Many experts suggest that patients who are taking antidepressants along with tamoxifen should talk over handling options with their doctors, such as switching from an SSRI that is a potent inhibitor of CYP2D6, such as paroxetine hydrochloride (Paxil), to one that is a weaker inhibitor, such as sertraline (Zoloft) or citalopram (Celexa), or to an antidepressant that does not inhibit CYP2D6, such equally venlafaxine (Effexor) (31). Or doctors may suggest that their postmenopausal patients accept an aromatase inhibitor instead of tamoxifen.
Other medications that inhibit CYP2D6 include the following:
- quinidine, which is used to treat abnormal center rhythms
- diphenhydramine, which is an antihistamine
- cimetidine, which is used to reduce breadbasket acid
People who are prescribed tamoxifen should discuss the use of all other medications with their doctors.
Source: https://www.cancer.gov/types/breast/breast-hormone-therapy-fact-sheet
0 Response to "Is Hormone Therapy Linked to Breast Cancer in Seniors Peer Reviews"
Post a Comment